Please wait.

Director Strategic Partner
Pfizer - Cheyenne, WY
Director Strategic Partner

Oops! This job has expired, but don’t worry.
Explore other exciting job listings and take the next step in your career journey!
137
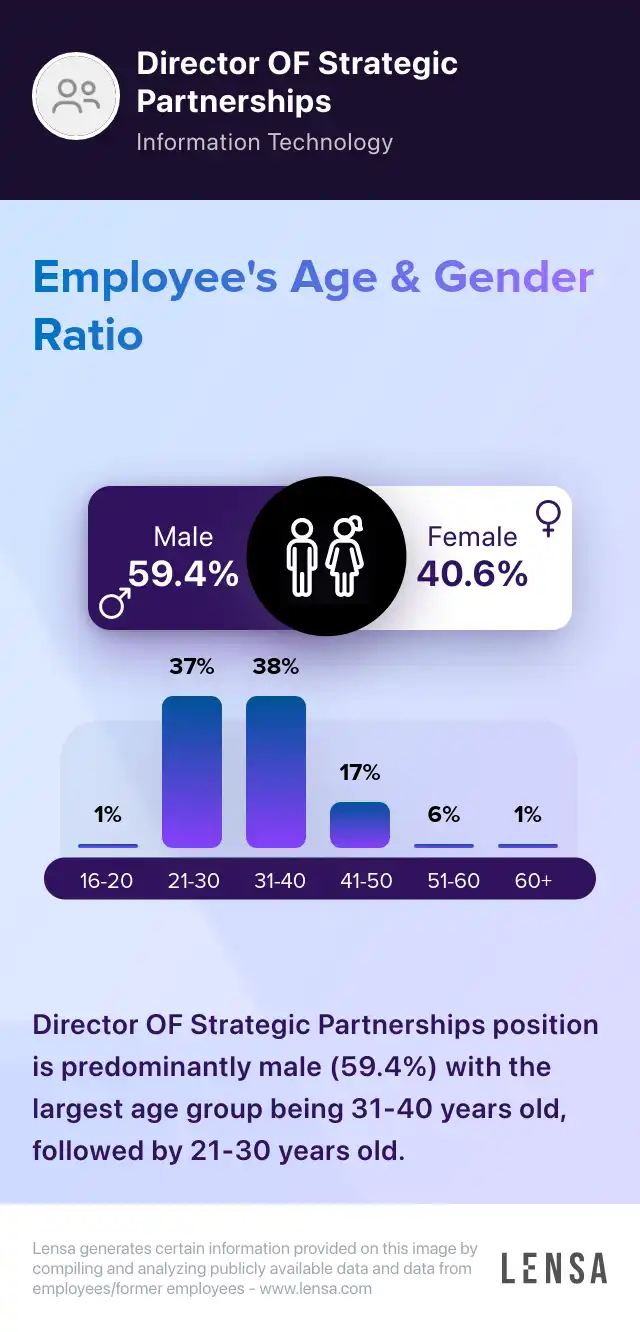
Director OF Strategic Partnerships
jobs978
jobs in
Cheyenne, WY
15
jobs at
Pfizer
Job
Company
Description
Salary
Skills
Benefits
Job Description
ROLE SUMMARY
Reporting to the Sr. Director Operations Management in Pfizer Global Supply (PGS) QO&EHS, the Strategic Partner role will lead the Strategy Development process for QO&EHS within PGS. PGS is transforming how it works to ensure our manufacturing network is prepared to meet the challenges of an evolving industry and the needs of all of our stakeholders. This role is critical to help enable QO&EHS to continue adapting how it delivers on its Fundamental Value Proposition of quality/compliance, cost/cash/value and supply reliability noting compromising on quality / compliance / safety is not an option.
This role will also lead the cross-functional and matrix-based strategy development in partnership and in collaboration with functions within PGS and across Pfizer as defined based on business needs.
ROLE RESPONSIBILITIES
-
Lead the development of the overall QO&EHS Digital strategy in line with Digital & technology investments and integration for the future of QO & EHS, aligned with operating unite leadership teams, key PGS functions.
-
Devise and execute the digital roadmap to make things as simple as possible and to deliver meaningful outcomes as it relates to the digital vision
-
Liaise with Digital to articulate, frame and prioritize the digital technology requirements to enable QO & EHS objectives. This includes the technical specification and assessment of new data science technologies
-
Lead the assessment of competitive intelligence as input into the overall strategy for assigned technology area.
-
Leads a variety of strategic projects including but not limited to defining strategies in line with the specificity and the complexity of overall initiatives and provide PMO expertise
-
Lead, develop as appropriate governance process to ensure robust execution and delivery on digital roadmap to enable QO&EHS transformation.
-
Instill a culture of ownership, leadership and management excellence. Keep a high level of engagement through open communication/dialog and empowerment
-
Ensure alignment and partnership with critical PGS enabling function such as external supply operations, engineering, technology functions and others within PGS
-
Promote cultural change whereby digital skills and tools are leveraged to drive higher productivity and performance as a preferred method for getting things done.
-
Collaborate with the Data Analytics Team, the PMO function and the Operational Excellence teams to identify opportunities that increase productivity and performance across the QO & EHS organization.
-
Capability and Talent development (early career talent RDE etc.)
-
Lead the assessment of competitive intelligence as input into the overall strategy for assigned technology area.
-
Leverage Quality Intelligence information and data analytics to challenge conventions, Champion Bold Thinking to improve QO&EHS performance.
-
Provide managerial guidance on goals and prioritization, issue escalation and coaching for performance.
-
Partner to develop and implement innovative technology strategies in partnership with PGS site and above-site functions, WRD (Bio and Small Molecule Pharmaceutical Sciences), Business Technology, Procurement, Legal, Finance, and external academic/technology/industry/engineering collaborators to deliver value to the PGS fundamental value proposition and GTS Value drivers: Platform Technology, Revenue Generation, Supply Assurance, Cost Reduction.
-
Active identification, prioritization, implementation, and replication of technology solutions in partnership with Operations, Quality, Pharm Sciences, Regulatory, and other internal/external functions.
BASIC QUALIFICATIONS
-
Bachelor's Degree
-
A minimum of 8 years' experience in a technical role in the biopharmaceutical manufacturing. Proficiency in process improvement methodologies, project management and digital applications.
-
Strong knowledge of quality and manufacturing operations
-
Proven ability to lead organizational change.
-
Strong analytical and strategic skills with experiencing solving complex and unique problems
-
Well-developed interpersonal skills with ability to collaborate across PGS functions and work effectively across all levels of the organization
-
Excellent written and verbal communications skills, and strong presentation skills. An ability to succinctly, clearly and compellingly present complex initiatives to colleagues and senior leaders.
-
Effective project management skills and timely deliverables.
-
Provide and facilitate strategic decision making to drive program and department decisions as a pathway to OpU success
-
Change Management Agent, fostering Continuous Improvement Culture
-
Strong ability to lead, challenge and positively influence in an interactive team environment.
-
Ability to effectively coach/mentor/influence colleagues and manage diverse relationships.
-
High degree of emotional intelligence, professional maturity, and credibility, with a proven record of successfully influencing key stakeholders and drive performance.
-
Candidate demonstrates a breadth of diverse leadership experiences and capabilities including: the ability to influence and collaborate with peers, develop and coach others, oversee and guide the work of other colleagues to achieve meaningful outcomes and create business impact.
PREFERRED QUALIFICATIONS/EXPERIENCE
- An advanced degree is preferred.
PHYSICAL/MENTAL REQUIREMENTS
-
Remains organized & positive in ambiguous and fast-paced, rapidly changing situations
-
Ability to analyze data from detailed schedule and risk management tools
-
Interface effectively with multiple stakeholder groups
-
Able to effectively assist with problem solving
NON-STANDARD WORK SCHEDULE, TRAVEL OR ENVIRONMENT REQUIREMENTS
-
10 - 15% travel
-
Flexible work hours to support a global operation
ADDITIONAL POSITION DETAIL
-
Last Date to Apply - Feb 9 2023
-
Position can be Remote
The annual base salary for this position ranges from $156,900.00 to $251,400.00. In addition, this position offers an annual bonus with a target of 20.0% of the base salary and eligibility to participate in our share based long term incentive program. Benefits offered include a retirement savings plan, paid vacation, holiday and personal days, paid caregiver/parental and medical leave, and health benefits to include medical, prescription drug, dental and vision coverage in accordance with the terms and conditions of the applicable plans. Salary range does not apply to the Tampa, FL location.
Relocation assistance may be available based on business needs and/or eligibility.
Pfizer requires all U.S. new hires to be fully vaccinated for COVID-19 prior to the first date of employment. As required by applicable law, Pfizer will consider requests for Reasonable Accommodations.
Sunshine Act
Pfizer reports payments and other transfers of value to health care providers as required by federal and state transparency laws and implementing regulations. These laws and regulations require Pfizer to provide government agencies with information such as a health care provider's name, address and the type of payments or other value received, generally for public disclosure. Subject to further legal review and statutory or regulatory clarification, which Pfizer intends to pursue, reimbursement of recruiting expenses for licensed physicians may constitute a reportable transfer of value under the federal transparency law commonly known as the Sunshine Act. Therefore, if you are a licensed physician who incurs recruiting expenses as a result of interviewing with Pfizer that we pay or reimburse, your name, address and the amount of payments made currently will be reported to the government. If you have questions regarding this matter, please do not hesitate to contact your Talent Acquisition representative.
EEO & Employment Eligibility
Pfizer is committed to equal opportunity in the terms and conditions of employment for all employees and job applicants without regard to race, color, religion, sex, sexual orientation, age, gender identity or gender expression, national origin, disability or veteran status. Pfizer also complies with all applicable national, state and local laws governing nondiscrimination in employment as well as work authorization and employment eligibility verification requirements of the Immigration and Nationality Act and IRCA. Pfizer is an E-Verify employer.
Bus Dev & Strategic Planning
#LI-PFE
This job was posted on Mon Jan 23 2023 and expired on Fri Feb 17 2023.
Find out how you match this company


Pfizer
Pharmaceutical Manufacturers
Reviews
Summary
Research-based biopharmaceutical company Pfizer aims to apply science and global resources to deliver innovative therapies that extend and significantly improve lives, challenging some of the most feared diseases of our time. The New York-headquartered company collaborates with healthcare providers, governments and local communities to support and expand access to reliable, affordable healthcare around the world. Pfizer believes that the strength of its network comes from the diversity of the team, and that different backgrounds, ideas and perspectives add valuable new dimensions to the common goal. If you are dedicated to pharmaceutical development and innovation, and would like to open new doors at Pfizer, you will find an array of career opportunities whether you are a postdoc, MBA student, undergrad or veteran. In case you find a role that suits your skills and talents, Pfizer will offer you competitive compensation and benefits based on a variety of factors including prior experience, geographic location, and talent pool availability.
How do you identify and cultivate strategic partnerships?
Answer
I identify potential partners through market research and networking. I then cultivate relationships by understanding their needs and aligning them with our organization's goals.
Can you provide an example of a successful strategic partnership you have developed?
How do you measure the success of strategic partnerships?

About the Director OF Strategic Partnerships role
PR Director OF Strategic Partnerships
As Director of Strategic Partnerships, we are responsible to deal with business relations, long term affiliates. We work together as a team to fulfill customer's objectives and goals by development of project management steps and tasks. To attract potential clients our mechanism of client cooperation is quick and smooth.
Core tasks:
- management of digital payments holding B2B and B2C customers
- management of sales and development by cold calling
- establishment of account management systems and customer relationship management structures
- development of sales processes and salesforce systems
137 Director OF Strategic Partnerships jobs in Cheyenne, WY

Similar jobs in the area